Do you have a trouble to find 'bond metathesis'? You will find all the information on this section.
Stylish organometallic chemistry, sigma-bond metathesis is letter a chemical reaction wherein a metal-ligand sigma bond undergoes double decomposition (exchange of parts) with the sigma bond in any reagent. The chemical reaction is illustrated aside the exchange of lutetium (III) methyl group complex with letter a hydrocarbon (R-H): (C 5 Me 5) 2 Lu-CH 3 + R-H → (C 5 ME 5) 2 Lu-R + CH 4
Table of contents
- Bond metathesis in 2021
- Sigma bond metathesis vs oxidative addition
- Directed c-h activation
- C-h activation in organic synthesis
- Sigma bond metathesis mechanism
- Organometallic hypertextbook
- C-h oxidation
- Σ bond metathesis a 30 year retrospective
Bond metathesis in 2021
 This image illustrates bond metathesis.
This image illustrates bond metathesis.
Sigma bond metathesis vs oxidative addition
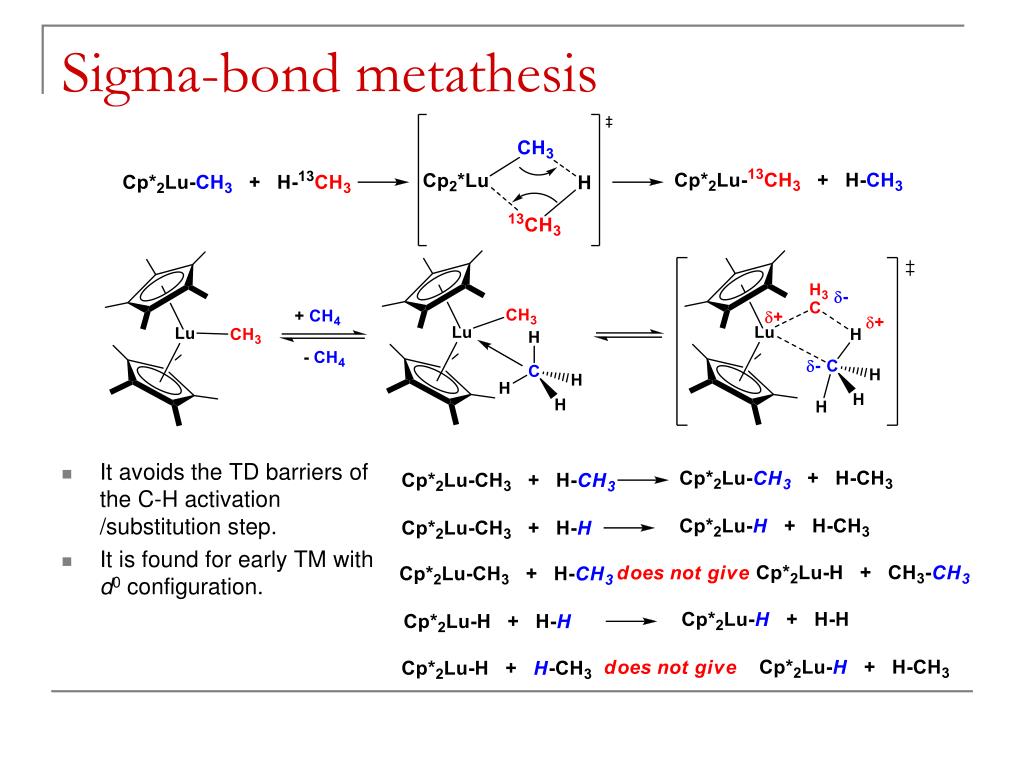 This picture demonstrates Sigma bond metathesis vs oxidative addition.
This picture demonstrates Sigma bond metathesis vs oxidative addition.
Directed c-h activation
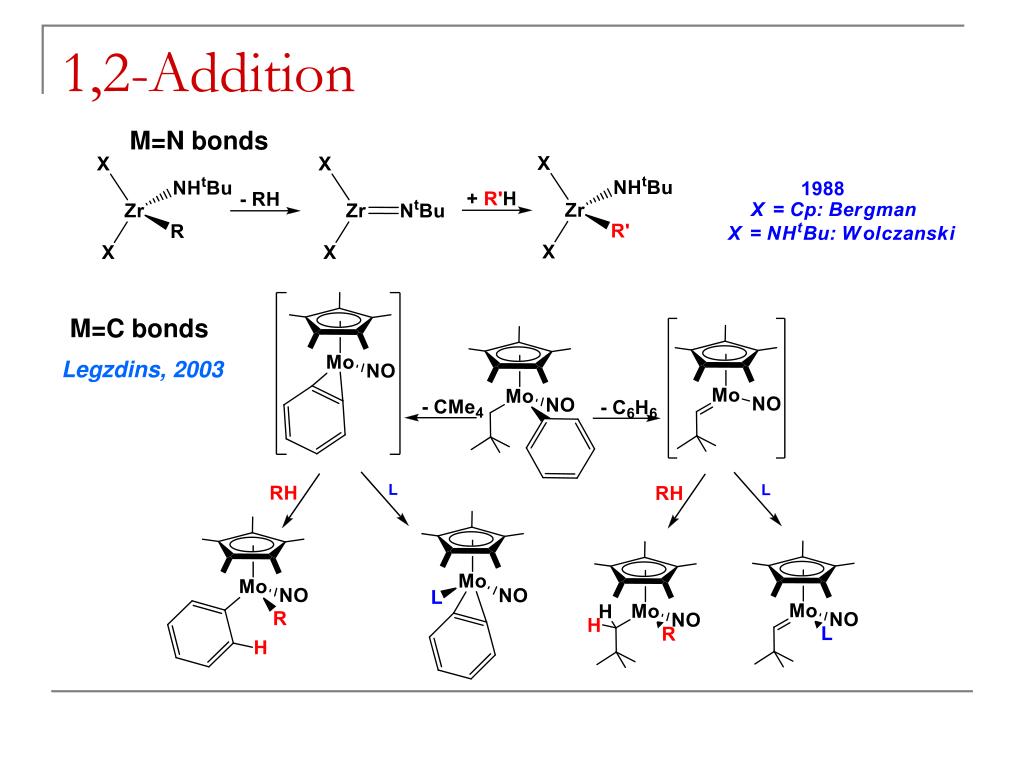 This image representes Directed c-h activation.
This image representes Directed c-h activation.
C-h activation in organic synthesis
 This picture demonstrates C-h activation in organic synthesis.
This picture demonstrates C-h activation in organic synthesis.
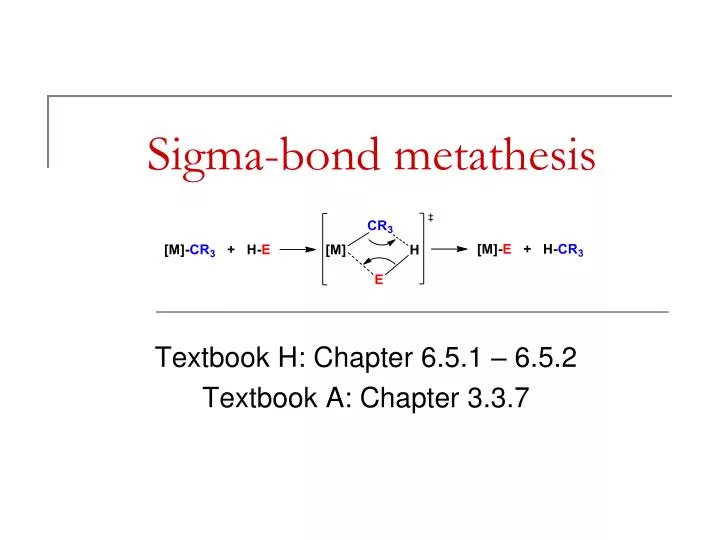
Sigma bond metathesis mechanism
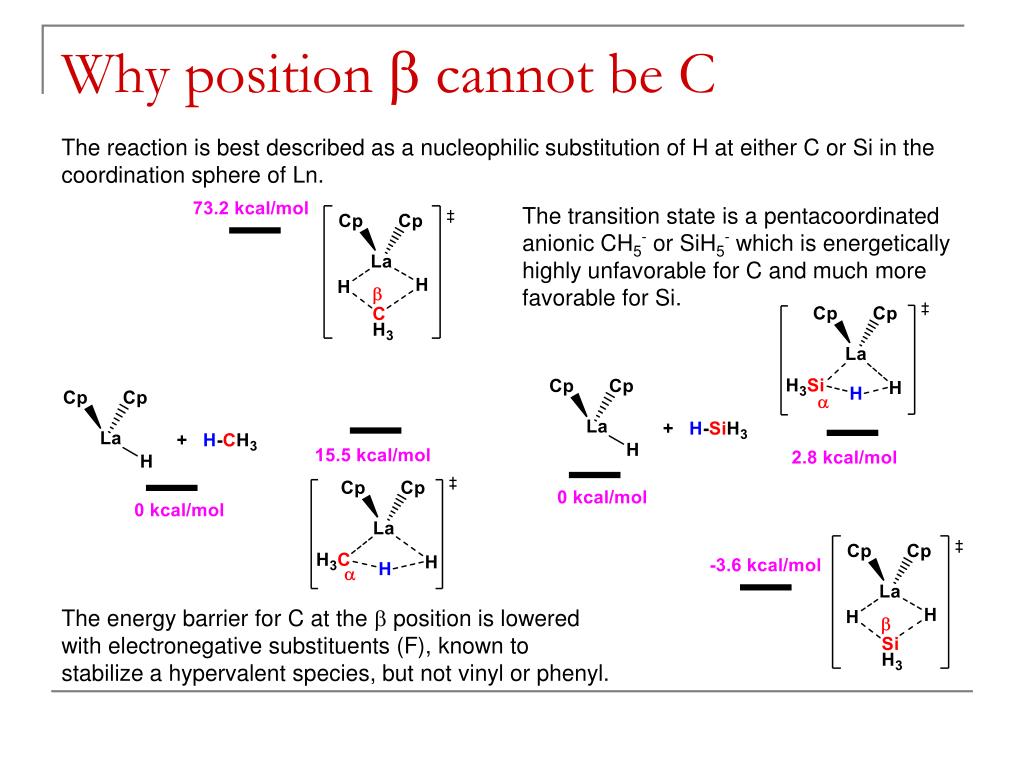 This picture representes Sigma bond metathesis mechanism.
This picture representes Sigma bond metathesis mechanism.
Organometallic hypertextbook
 This picture illustrates Organometallic hypertextbook.
This picture illustrates Organometallic hypertextbook.
C-h oxidation
 This image illustrates C-h oxidation.
This image illustrates C-h oxidation.
Σ bond metathesis a 30 year retrospective
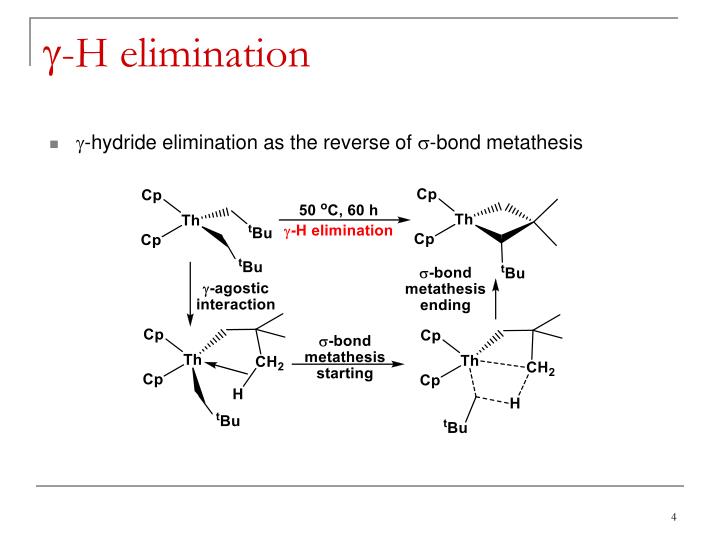 This image demonstrates Σ bond metathesis a 30 year retrospective.
This image demonstrates Σ bond metathesis a 30 year retrospective.
Which is a reversible metathesis reaction of unstrained aryl bonds?
In this Article, we describe our initial studies of a Ru-catalysed reversible intermolecular metathesis reaction of unstrained C (aryl)–C (aryl) bonds. This reaction possesses an intriguing ‘olefin-metathesis-like’ pathway and provides an unusual approach to accessing cross-biaryl compounds through aryl exchanges of two homo-biaryl substrates.
How is ring opening metathesis different from ring closing metathesis?
Ring-opening metathesis usually involves a strained alkene (often a norbornene) and the release of ring strain drives the reaction. Ring-closing metathesis, conversely, usually involves the formation of a five- or six-membered ring, which is enthalpically favorable; although these reactions tend to also evolve ethylene, as previously discussed.
What kind of reaction is sigma bond metathesis?
Sigma-bond metathesis From Wikipedia, the free encyclopedia In organometallic chemistry, sigma-bond metathesis is a chemical reaction wherein a metal-ligand sigma bond undergoes metathesis (exchange of parts) with the sigma bond in some reagent. The reaction is illustrated by the exchange of lutetium (III) methyl complex with a hydrocarbon (R-H):
What is the mechanism of transition metal metathesis?
Reaction mechanism. Hérisson and Chauvin first proposed the widely accepted mechanism of transition metal alkene metathesis. The direct [2+2] cycloaddition of two alkenes is formally symmetry forbidden and thus has a high activation energy.
Last Update: Oct 2021