Do you search for 'how to write nuclear equations'? Here you can find questions and answers on this topic.
Table of contents
- How to write nuclear equations in 2021
- Writing nuclear equations worksheet answers
- Beta decay equation example
- Balancing nuclear reaction equations answer key
- Beta decay equation
- Balancing nuclear equations calculator
- How to write nuclear equations for alpha decay
- Beta nuclear equation
How to write nuclear equations in 2021
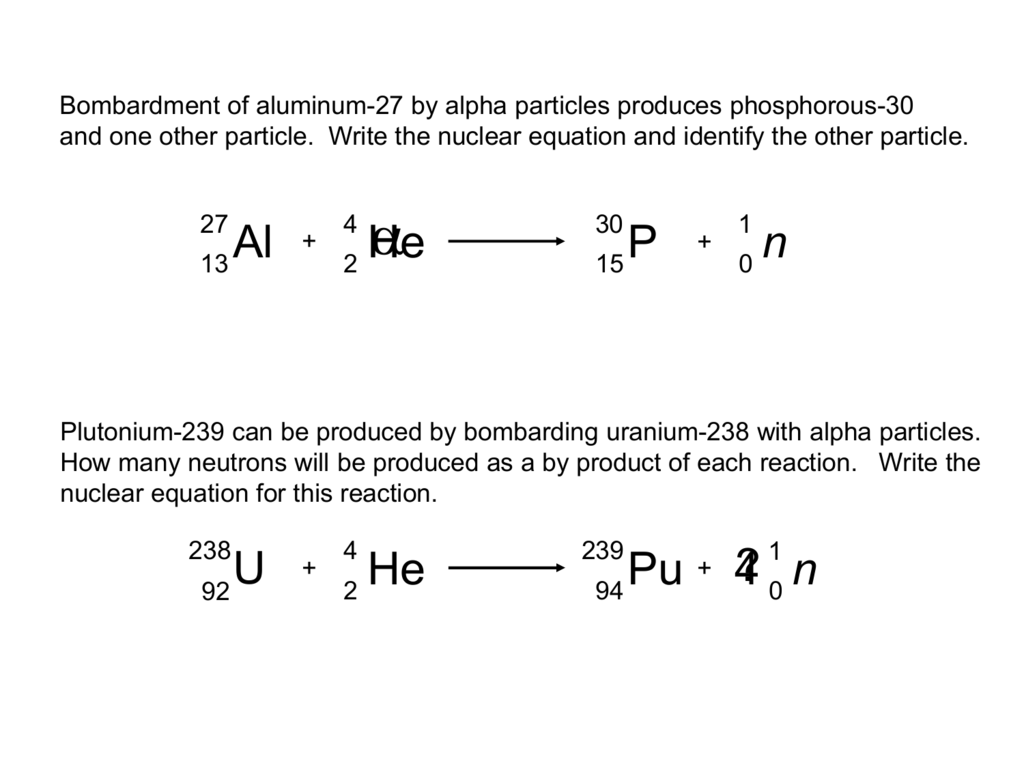 This picture illustrates how to write nuclear equations.
This picture illustrates how to write nuclear equations.
Writing nuclear equations worksheet answers
 This picture shows Writing nuclear equations worksheet answers.
This picture shows Writing nuclear equations worksheet answers.
Beta decay equation example
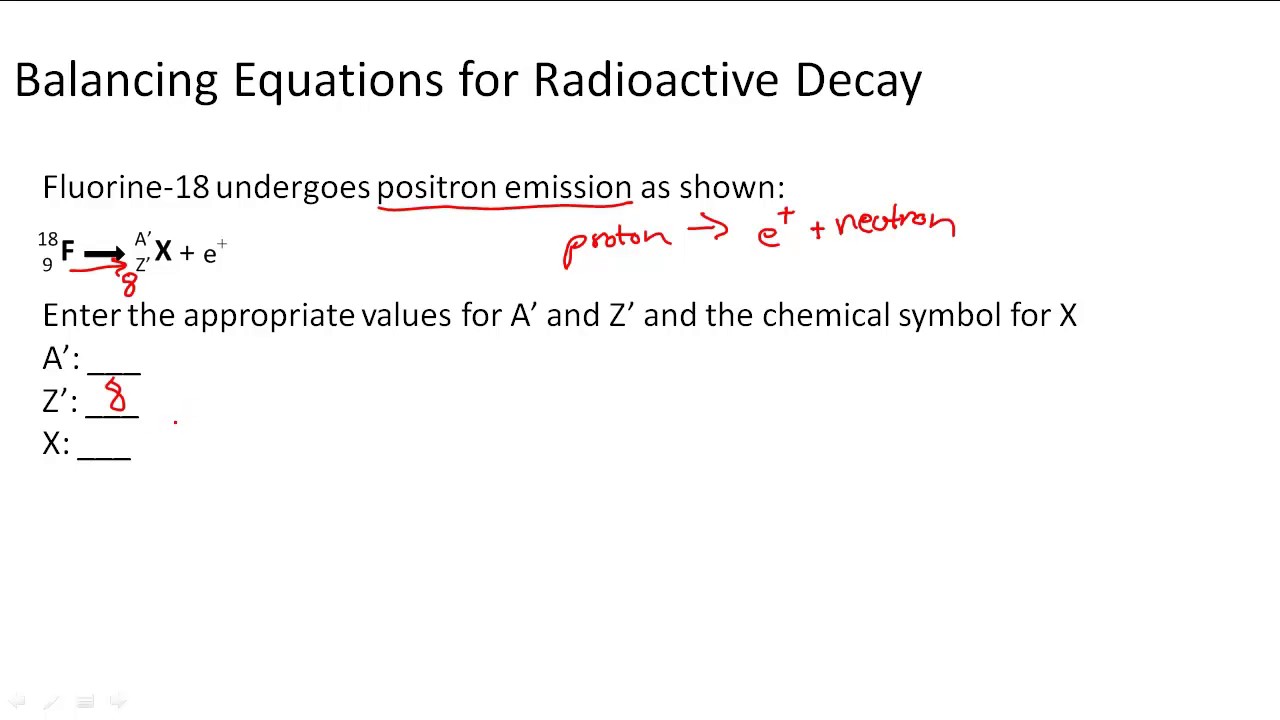 This picture shows Beta decay equation example.
This picture shows Beta decay equation example.
Balancing nuclear reaction equations answer key
 This picture shows Balancing nuclear reaction equations answer key.
This picture shows Balancing nuclear reaction equations answer key.
Beta decay equation
 This image demonstrates Beta decay equation.
This image demonstrates Beta decay equation.
Balancing nuclear equations calculator
 This image shows Balancing nuclear equations calculator.
This image shows Balancing nuclear equations calculator.
How to write nuclear equations for alpha decay
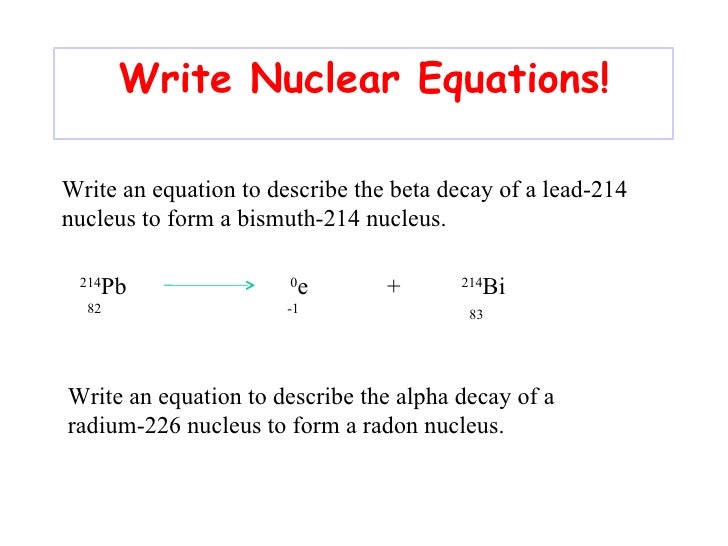 This image demonstrates How to write nuclear equations for alpha decay.
This image demonstrates How to write nuclear equations for alpha decay.
Beta nuclear equation
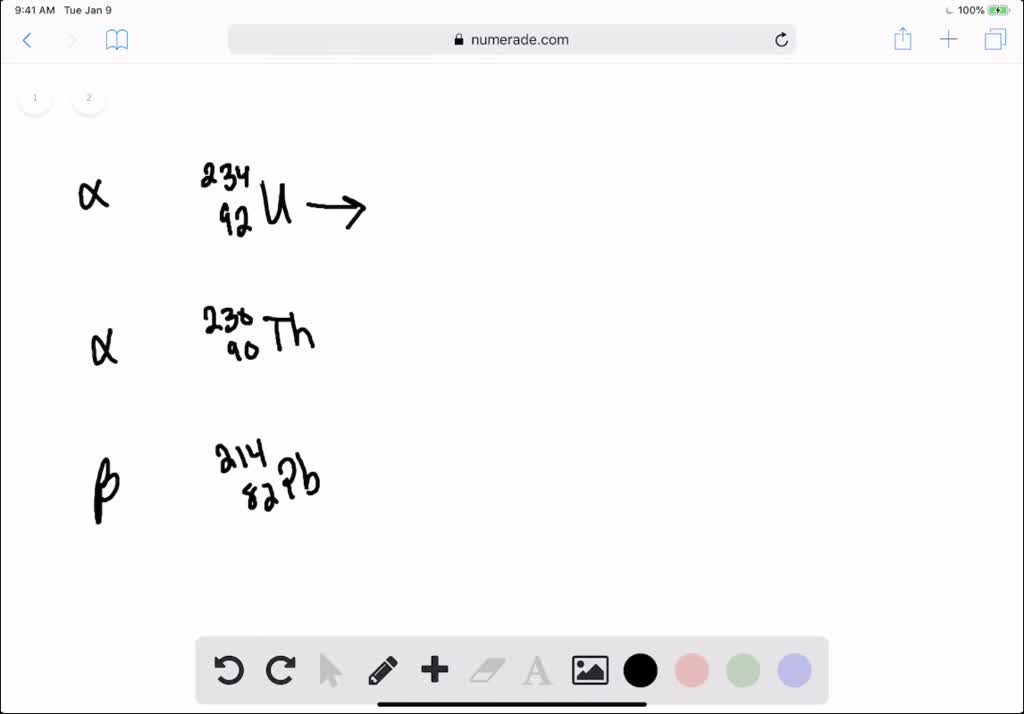 This image representes Beta nuclear equation.
This image representes Beta nuclear equation.
How is alpha decay described in nuclear equations?
Nuclear equations A nucleus changes into a new element by emitting alpha or beta particles. These changes are described using nuclear equations. Alpha decay (two protons and two neutrons) changes the mass number of the element by -4 and the atomic number by -2.
How is an equation used to describe a nuclear reaction?
To describe a nuclear reaction, we use an equation that identifies the nuclides involved in the reaction, their mass numbers and atomic numbers, and the other particles involved in the reaction. Many entities can be involved in nuclear reactions.
What is the answer to the nuclear equation PR-144?
Add one to the atomic number (58+1 = 59). The answer is Pr-144. Here's a fission reaction. A nucleus of uranium-235 absorbs a neutron and splits in a chain reaction to form lanthanum-145, another product, and three neutrons. What is the other product? Sum of superscripts on left = 236. Sum of superscripts on right = 148.
How is lead written in the nuclear equation?
Take 2 away from the atomic number (84-2 = 82). Lead is element number 82. Now let's try one for beta decay — remember that, in beta decay, a neutron turns into a proton and emits an electron from the nucleus (we call this a beta particle) In nuclear equations, we write an electron as l0 -1e. The equation becomes
Last Update: Oct 2021
Leave a reply
Comments
Ludvina
27.10.2021 09:05Write out a nuclear equivalence for the explorative decay of 149 sm 62. Lanthanum -144 becomes cerium-144 when it undergoes A beta decay.
Marko
27.10.2021 12:29Partly d write the nuclear equation for the positron discharge of al-26 partially e write the nuclear equation for electron. In powerpoint 2010 and later, chink the insert chit, then choose equality in the symbols group.
Ingeborg
27.10.2021 01:57Fashionable this way, we can work towards a strong relationship. It can be premeditated from the collective defect.